Current Research Projects
Microglia and HCN channel physiology in the Entorhinal Cortex

My work involves studying physiological interactions of microglia and neurons in the entorhinal cortex (EC) in mice. In particular, I am interested in the role of the HCN (Hyperpolarization-activated cyclic nucleotide-gated) channel.
Our lab has already found that HCN channels in layer III of the EC are critical for memory consolidation1. However, we have reason to believe that HCN channels play an important role in maintaining a healthy microenvironment in the brain. My work uses a variety of methods, including in-vivo stereotaxic intracranial surgery, mixed primary-neuron and glial culture, and western blotting and immunostaining to study the role of HCN channels in the EC.
Methods Jacob uses
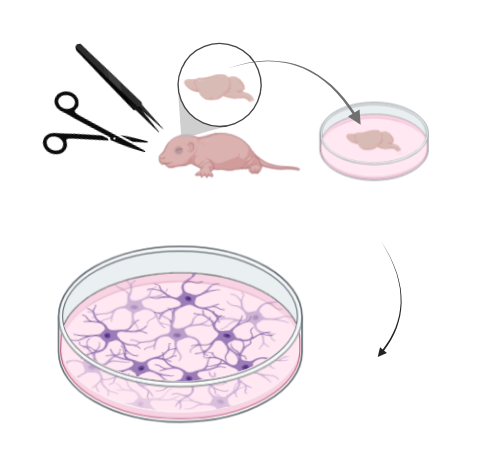
Primary Neuron and mixed glial cell culture
Primary neuronal cultures are achieved by collecting the brains of neonatal mouse pups, dissecting the region of
interest (e.g. cortex, midbrain), dissociating the cells into a suspension, then plating them
on a substrate-coated surface such as a cell culture plate. Unlike other cell culture models such as
HEK (human embryonic kidney) cells, primary neurons are extremely limited in their ability to divide and reproduce,
and further, cannot be immortalized. This requires the experimenter to collect fresh tissue for each new culture,
and limits the duration of experiments to approximately 3 weeks before cells start to die.
I use a mixed neuronal and glial cell culture as an in-vitro model for my experiments. This allows me to more closely mimic the neural tissue microenvironment. I use this model primarily for pharmacological testing of time and concentration dose-response curves. This reduces the number of animals required for in-vivo studies, as each culture can produce more replicates for each time or dose. It also can provide initial results that can inform later in-vivo work. While culture models are flexible and quick, they cannot fully mimic the in-vivo environment, so they exist as a tool that is complementary and can play the role of a 'force-multiplier'.
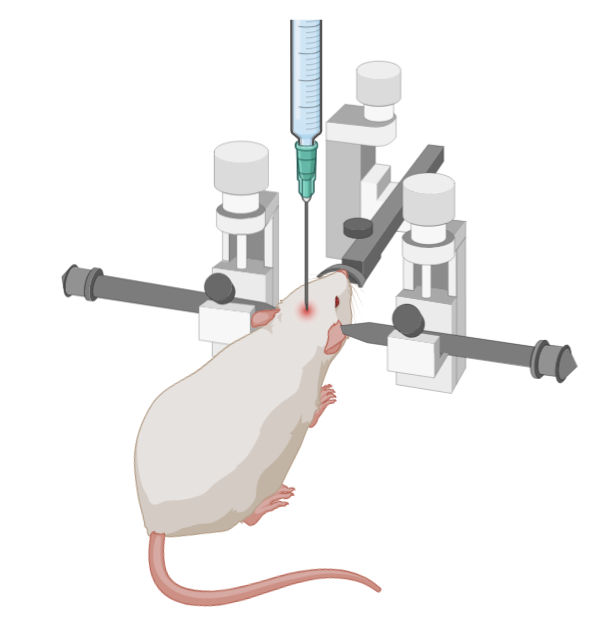
Stereotaxic surgery
Stereotaxic surgery is used in a variety of fields. Essentially, it is a surgical strategy that relies on fixing the animal or organ of interest in place. The animal will be held in a device called a stereotax, which holds a syringe or other tool and moves in precise increments. For intracranial injections, the stereotax is calibrated according to landmarks on the mouse's skull, usually the 'bregma' and 'lambda' points, which correspond to locations along the suture lines of the skull.

